
Sugars

Training Center Manager
July 30, 2025
CategoryEclair Technique
Sugars are one of the main ingredients in our sweet treats: pastries, confectionery, ice cream...
These molecules not only give finished products their sweet taste, but also have an impact on their structure and shelf life by influencing various parameters:
- Product color
- Water binding: action on drying out, brittleness, softness and crystallization, syneresis, moisture regain
- Dry extract
- The development of microorganisms, both bacteria and fermenters, and therefore pasta volume
Sugars are part of the carbohydrate family, and can be made up of molecules of varying complexity. Their energy value is 4kcal/g.
When their name ends in -ose, they are monosaccharides or disaccharides (meaning they are made up of one or two molecules).
| Class (depending on chain length) | Subgroup | Main carbohydrates |
|---|---|---|
| Polysaccharides | Starch | Amylose, amylopectin |
| Non-starch | Cellulose, pectin, inulin, galactomannans | |
| Oligosaccharides | Malto-oligosaccharides | Maltodextrins |
| Other oligosaccharides | More complex sugars | |
| Sugars | Monosaccharides | Glucose, galactose, fructose |
| Disaccharides | Sucrose, lactose, trehalose, maltose | |
| Hydrogenated glucose | Polyols |
Sucrose
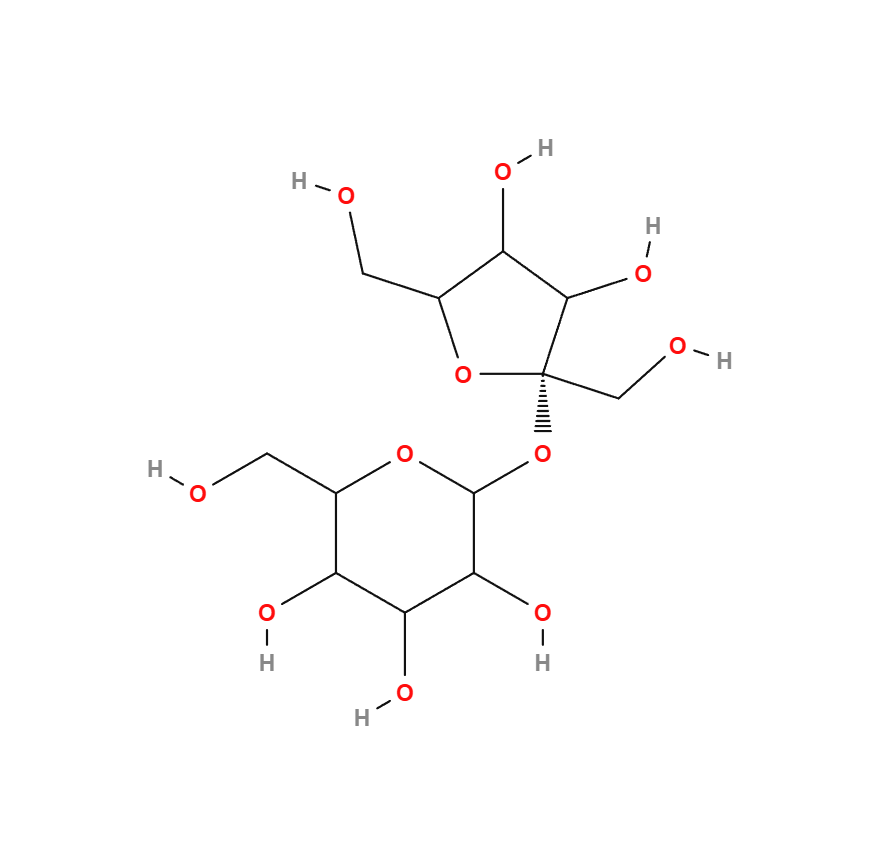
Composition: one glucose + one fructose
Sweet flavour: 1 or 100
Molecule making up commonly used white sugar (generally beet-based), more or less refined (brown sugar, vergeoise, etc.). It is also present in cane sugar (including muscovado and rapadura), coconut blossom sugar and maple syrup. Low-refined or unrefined sugars (brown sugars) provide fiber, trace elements and minerals. Some contain a little protein.
As soon as water is present, the molecule hydrolyzes and splits into fructose and dextrose (another name for glucose). The wetter and more heated sucrose is, the more it transforms into invert sugar, which can only be in paste form. The sweetness is thus more intense, close to fructose at 1.3 (or 130), and dextrose expresses its properties. Hygroscopicity is also greater, resulting in undesirable moisture pick-up in products such as fruit pastes. Cooking can intensify this inversion, impacting on taste, color and texture.
Both are present in honey:
- The greater the quantity of invert sugar in relation to sucrose, the more fluid the honey and the greater the sweetness.
- Conversely, the greater the quantity of sucrose in relation to invert sugar, the more compact the honey and the less intense the sweetness.
Starch derivatives
They are obtained from starch by hydrolysis: starch, which is a chain of glucoses, is cut into chains of varying lengths. Depending on the length of their constituent chains, starch derivatives are characterized by a D.E. (Dextrose Equivalent) value. This value is used to categorize them:
- DE 0 : starch
- DE ≤ 20 : maltodextrins (mixture of chains of 2, 3, 4... glucoses)
- DE > 21: glucose syrups (mixtures of glucose, maltose, trioses, etc.). The higher the DE, the higher the maltose and glucose content.
- DE 100: pure glucose (also known as dextrose)
Because of these different maltose and glucose contents, the properties of glucose syrups will vary: it is therefore important to specify their DE in recipes.
As starch derivatives are derived from glucose chains, they do not contain fructose.
Dextrose
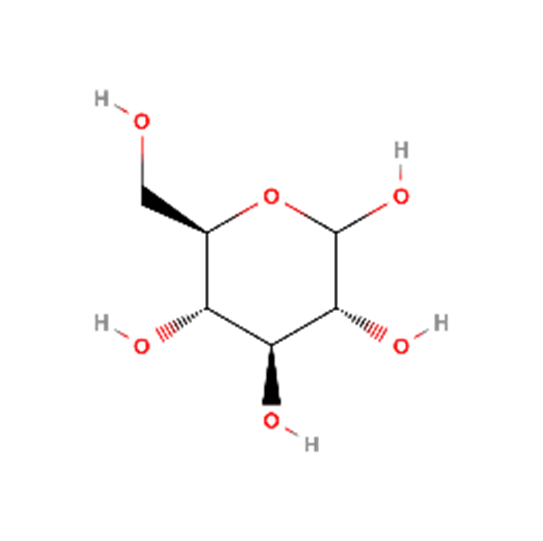
Another name for the glucose molecule.
As the smallest carbohydrate molecule, it solubilizes very quickly and the reaction consumes energy, making it feel cold when tasted.
Often present in the composition of carbohydrates, it enables solubilization.
Sweet: 0.75 or 75
Dextrose increases color (cookies, viennoiserie...) and improves bread-making volume (nutrient directly assimilated by yeast).
It has a high affinity with water, so improves softness (cakes, madeleines, sponge cakes...) and adds shine to toppings and glazes. It does, however, promote moisture absorption (making confectionery sticky).
It is an anti-crystallizing agent (it reduces the size of water crystals during freezing) and lowers the freezing point of ice creams, sorbets...
It's also an anti-crystallizing agent for sucrose crystallization, giving a fluid, transparent cooked sugar (the sugar doesn't lump, so hard candies don't whiten or crumble).
Glucose syrups
Mixtures of glucose (dextrose), maltose (2 glucoses), trioses (3 glucoses) and other glucose chains in different proportions according to D.E. Dextrose Equivalent. We'll concentrate on the most commonly used.
Glucose syrup DE40
It can be sulphited or unsulphured:
- Sulphite syrup is only used for cooked sugar. High-temperature cooking allows sulfites to turn into gas and no longer be present in the finished product, which is important given that sulfites are among the major allergens.
- Unsulphited syrup is suitable for all applications.
Glucose syrup DE60
Contains almost 50% dextrose and maltose. It provides the same amount of glucose as invert sugar, and therefore all the properties of interest to the professional, while limiting the sweetness by more than half .
Powdered glucose syrups
We use them in products where we want to limit the addition of water (ice creams, ganaches, etc.).
- DE25 : contains little free glucose but remains soluble while adding more viscosity to the preparation, which is interesting for sorbets based on very liquid fruit purées (citrus ) or ice creams with alcohol.
- DE40 : ideal for sorbets and ice creams because it contains enough free glucose to reduce ice crystals, considerably lower freezing point, and provide little sweetness while enhancing aromas.
Maltodextrins
Maltodextrins are the longest molecules after starch. Their properties are as follows:
- They add viscosity
- They have little or no sweetness
- They are used as dispersants, often as a carrier for powdered flavorings or colorants.
- They are present in most icing sugars because they are not very hygroscopic.
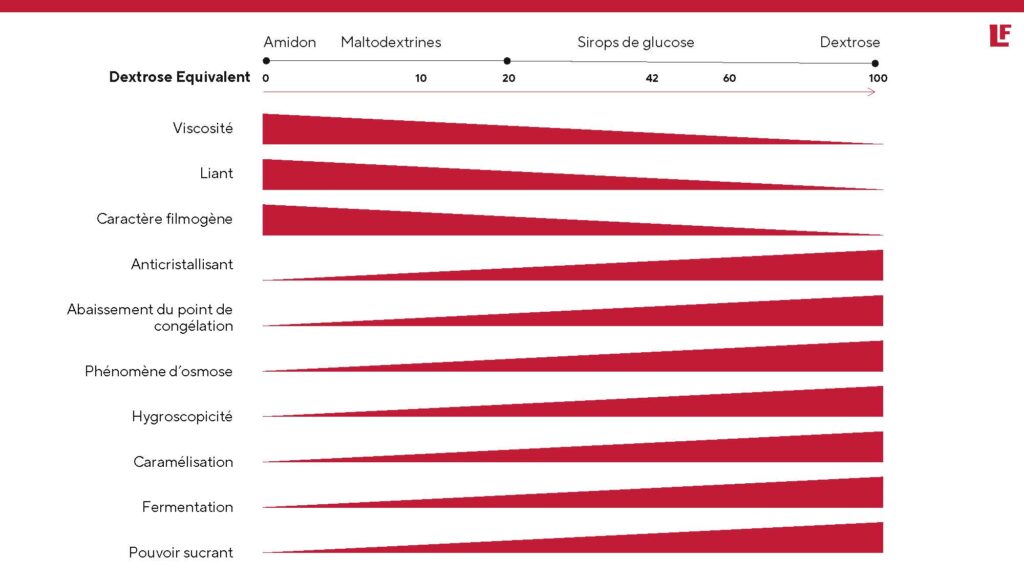
Sugar properties as a function of equivalent dextrose
The higher the Dextrose Equivalent (D.E.), the more :
- Rapid solubilization
- Caramelization , and therefore coloring, is important.
- The product is hygroscopic (high affinity with water)
- It is easily assimilated by yeasts, thus promoting fermentation.
- The ingredient is anti-crystallizing








